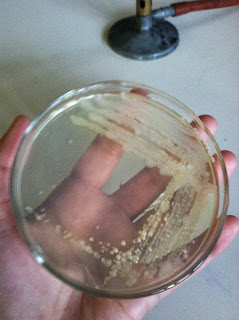
When we came back to class, we removed it from the incubator and were delighted to see a pure colony! This means that like bacteria will colonize together in little dots, which you can see in these pictures.
Our next step was to collect a bead of bacteria and put it on a microscope slide so we could observe the bacteria in greater detail.
Using the inoculating loop we grabbed a bead of the bacteria, after we sterilized the loop of course. On the microscope slide we gently mixed the bacteria with a small drop of de-ionized water. Then we waited for the slide to air dry. Once it was completely dry we heat fixed the culture to the slide. To do this we passed the slide quickly through the flame three times. Now it is ready to be Gram stained!
The bacteria that we viewed were all a consistent rod shape and were also all gram negative. This ensured that we finally obtained a pure culture from our original environmental sample.
We could deduce that our bacteria was gram negative because of the pink hue they all contain. If our bacteria was gram positive the coloring would have been purple. This happens because a gram positive cell has more peptidoglycan and is therefore more resistant to taking the stains in to its membrane.
Our next step was to collect a bead of bacteria and put it on a microscope slide so we could observe the bacteria in greater detail.
Using the inoculating loop we grabbed a bead of the bacteria, after we sterilized the loop of course. On the microscope slide we gently mixed the bacteria with a small drop of de-ionized water. Then we waited for the slide to air dry. Once it was completely dry we heat fixed the culture to the slide. To do this we passed the slide quickly through the flame three times. Now it is ready to be Gram stained!
We placed our slide on a drying rack over the sink at our lab bench. First we covered our smear with Crystal Violet for 20 seconds, and then rinsed the slide with distilled water to remove excess stain. Next we covered the smear with Gram's Iodine for one minute, and proceeded to rinse it with water afterwards to remove excess solution. After we held the slide at a 45 degree angle while adding EtOH until the color stopped running. We rinsed the slide immediately afterwards to remove the decolorizing agent. And lastly we covered the smear with Safranin for one minute, and then rinsed it with distilled water to remove the access Safaranin. Then to dry the slide we blotted the slide with a piece of bibulous paper.
Now our slide is ready to be viewed under a microscope using the oil immersion lens.
The bacteria that we viewed were all a consistent rod shape and were also all gram negative. This ensured that we finally obtained a pure culture from our original environmental sample.
We could deduce that our bacteria was gram negative because of the pink hue they all contain. If our bacteria was gram positive the coloring would have been purple. This happens because a gram positive cell has more peptidoglycan and is therefore more resistant to taking the stains in to its membrane.






No comments:
Post a Comment